Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa
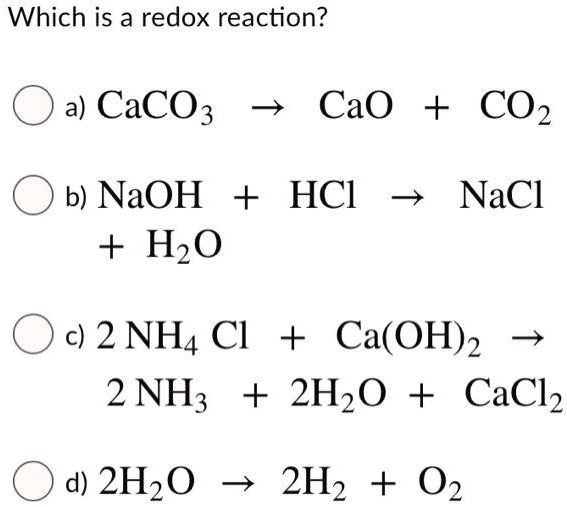
SOLVED: Which is a redox reaction? a) CaCO3 CaO + CO2 b) NaOH + HzO HCl NaCl c) 2 NH4 Cl + Ca(OH)2 2 NH; + 2Hz0 + CaClz d) 2Hz0 2H2 + 02
CO2 mineralization into different polymorphs of CaCO3 using an aqueous-CO2 system - RSC Advances (RSC Publishing)
8.CaCO3(s)=CaO(s) +CO2(g).If Kp for the above reaction at 1200K is 1.5 atm.Some CaCO3 is taken in a 10 litre closed vessel,then the number of moles of CaO at equilibrium is (Take R=1/12
![CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is CaCO3→ CaO + CO2,Δ H = + 180KJ . If entropies of CaCO3,CaO and CO2 respectively are 93,39 and 213J/mol/K , then Δ S (total) [in J/K] at 300K is](https://dwes9vv9u0550.cloudfront.net/images/2234632/d247a5dd-b799-4377-806f-2628447e070f.jpg)

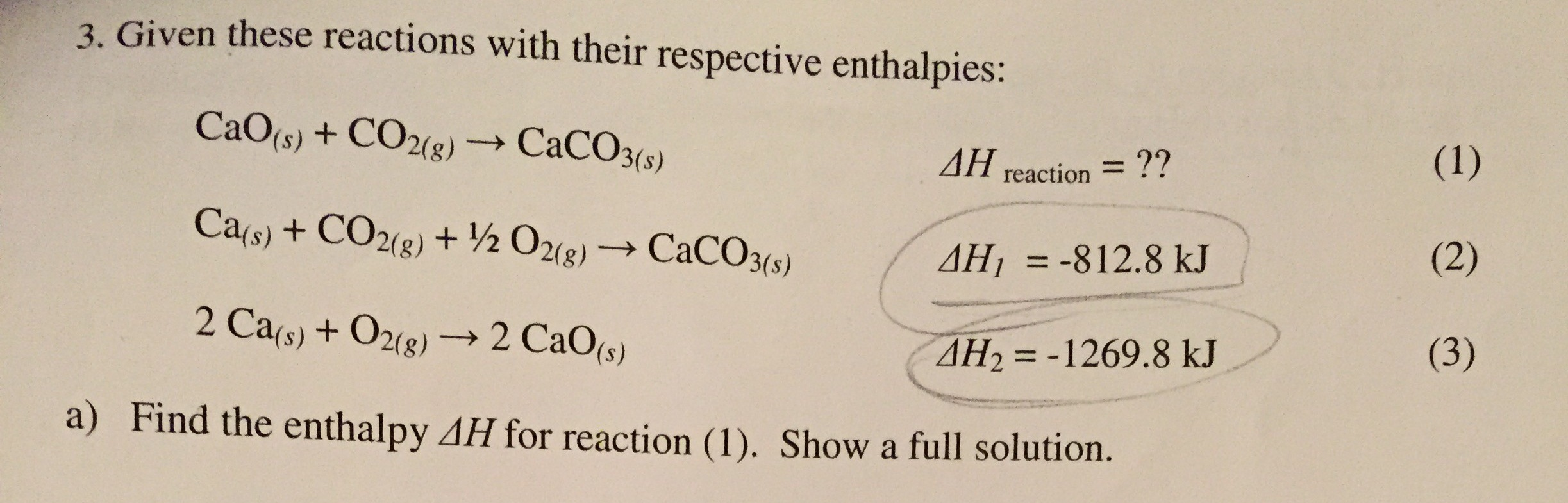
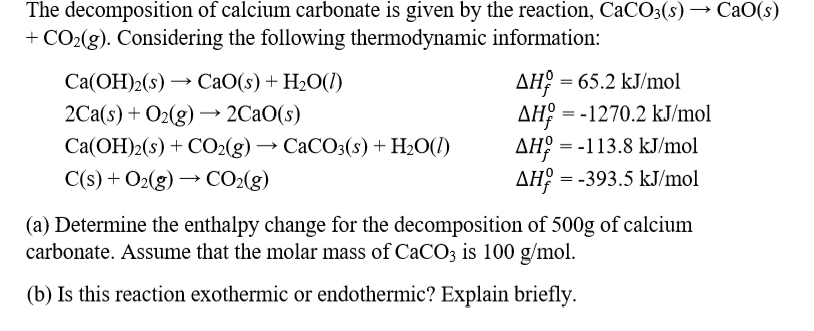

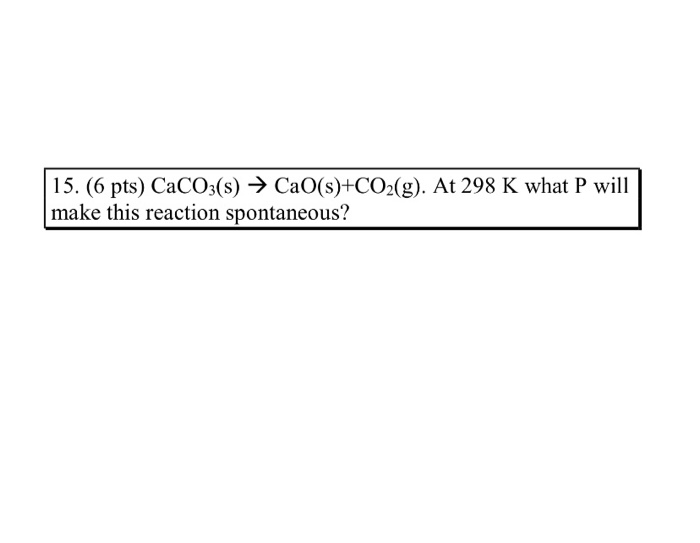



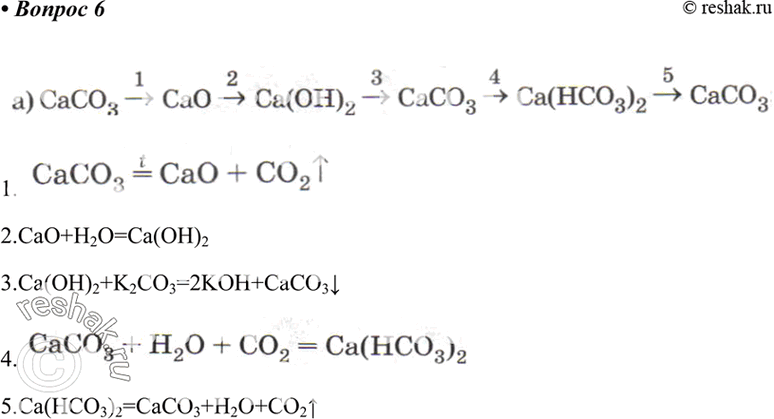





![PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar PDF] Energy analysis of CaCO3 calcination with CO2 capture | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/70ca22dce93c5e0c4e267a02ccc4e41951e44132/2-Figure2-1.png)