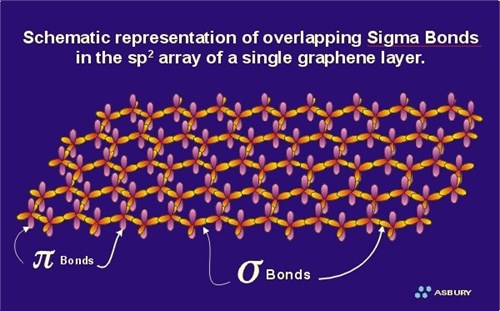
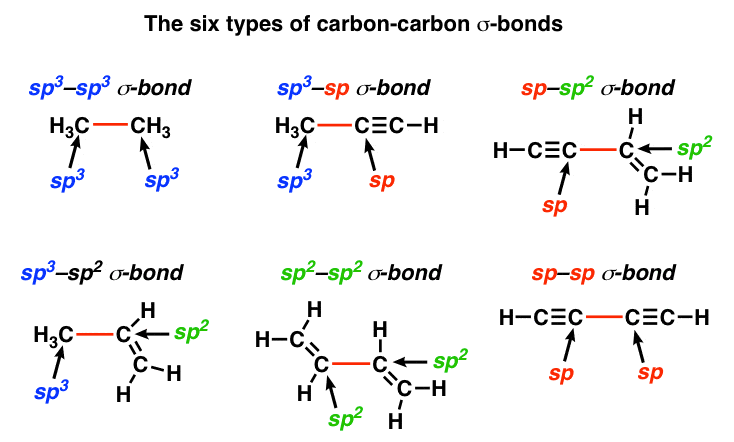
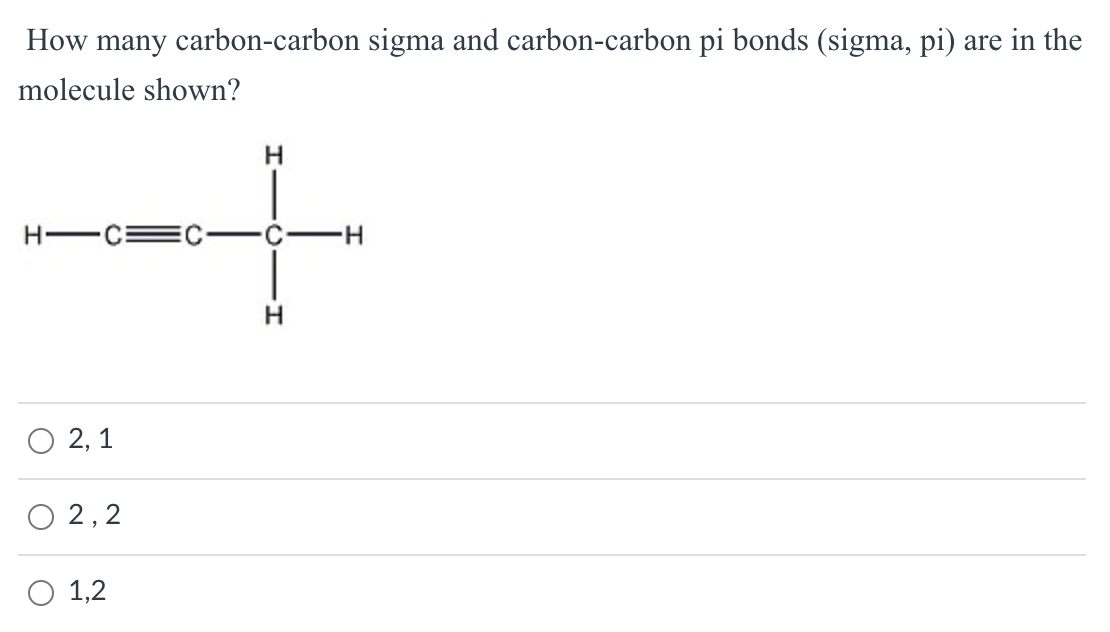
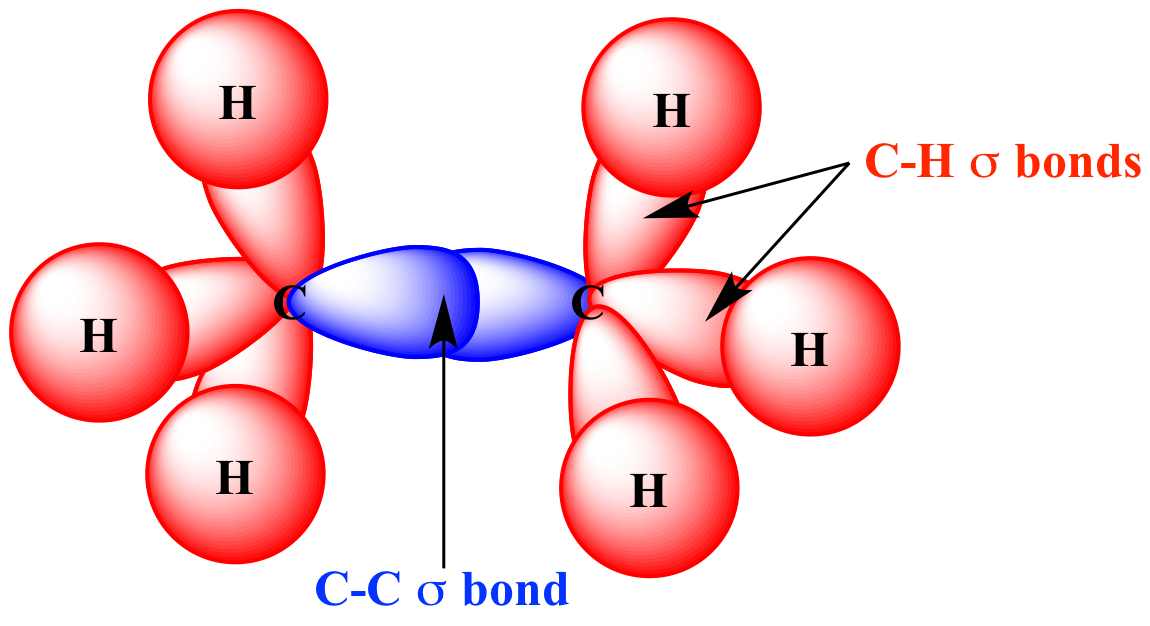
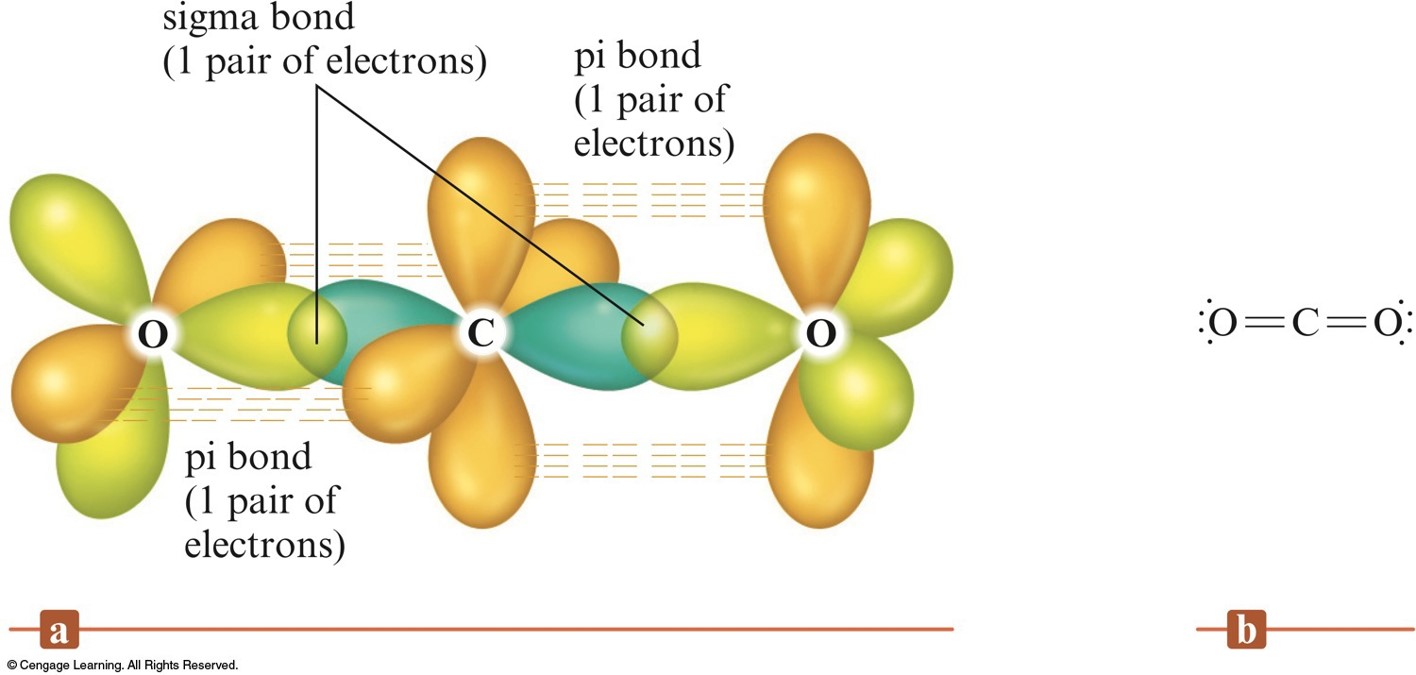
Which types of bonds are present between two carbon atoms in an acetylene molecule?Two sigma bonds and one pi bondThree pi bondsOne sigma bond and two pi bondsThree sigma bonds
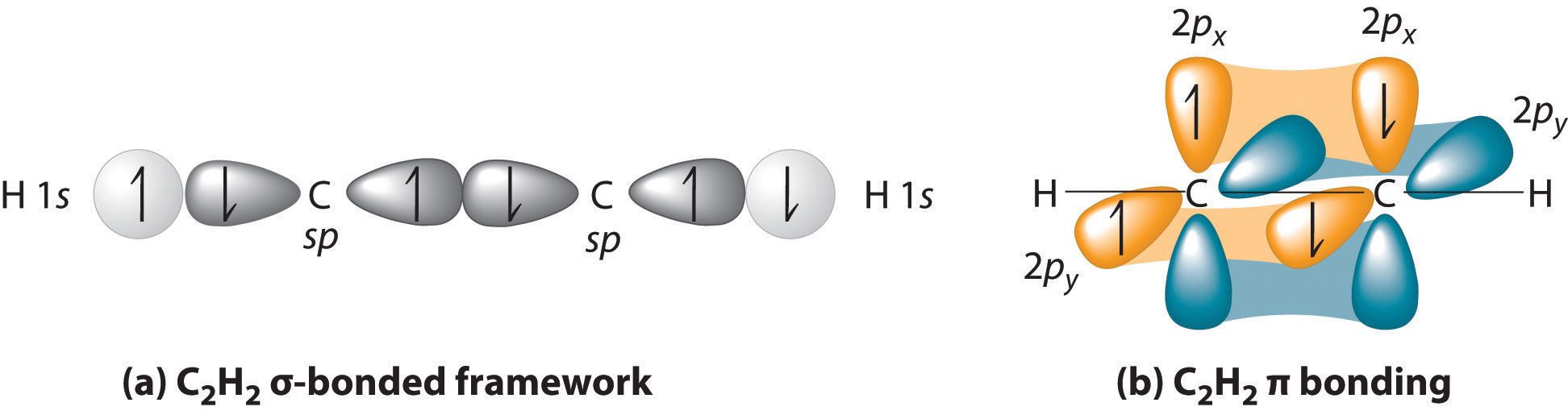
How does carbon use its "s" and "p" orbitals to form bonds in ethyne, ethene, and ethane? | Socratic

How many carbon-carbon Sigma bonds are in the molecule shown? a) 1 b) 2 c) 3 d) 4 | Homework.Study.com

1.7.3.a. state the general formula of alkenes and understand that they are unsaturated hydrocarbons with a carbon-carbon double bond which consists of a sigma and a pi bond Flashcards | Quizlet
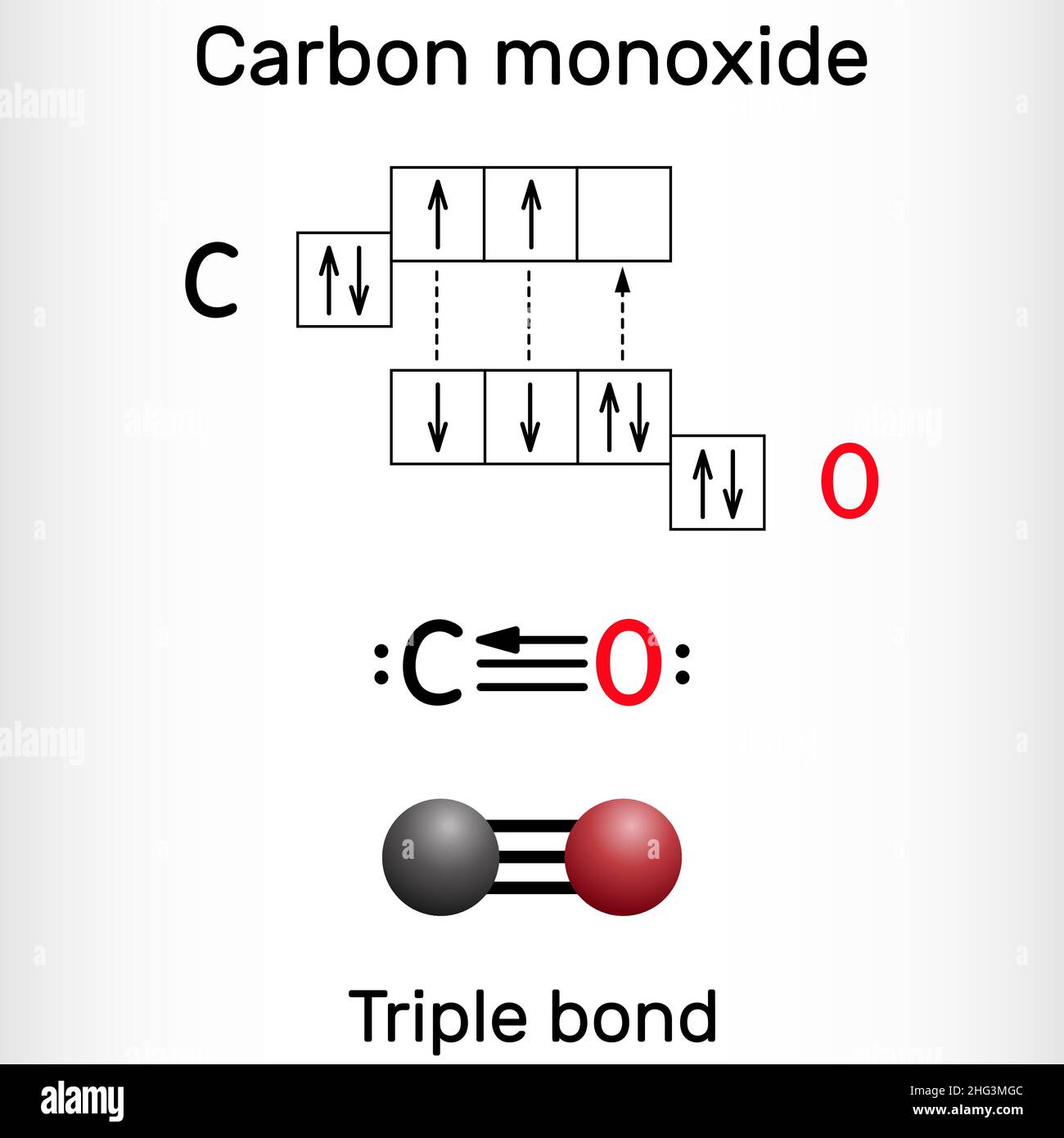
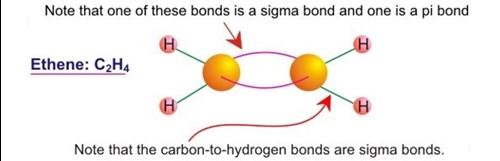
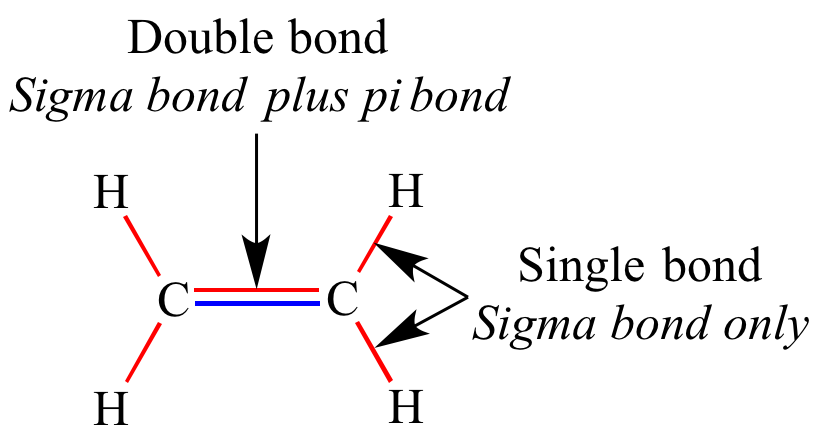


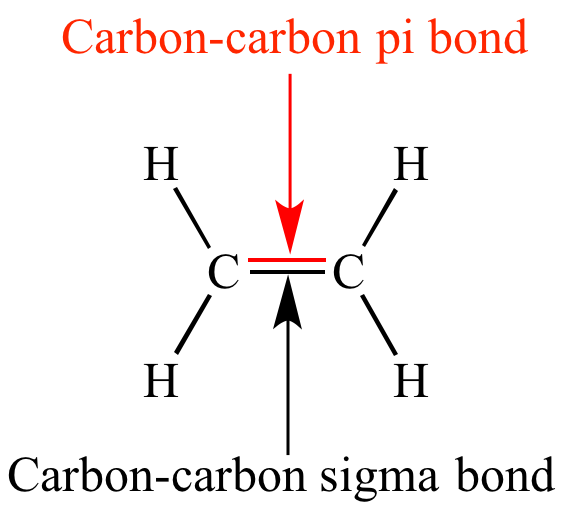


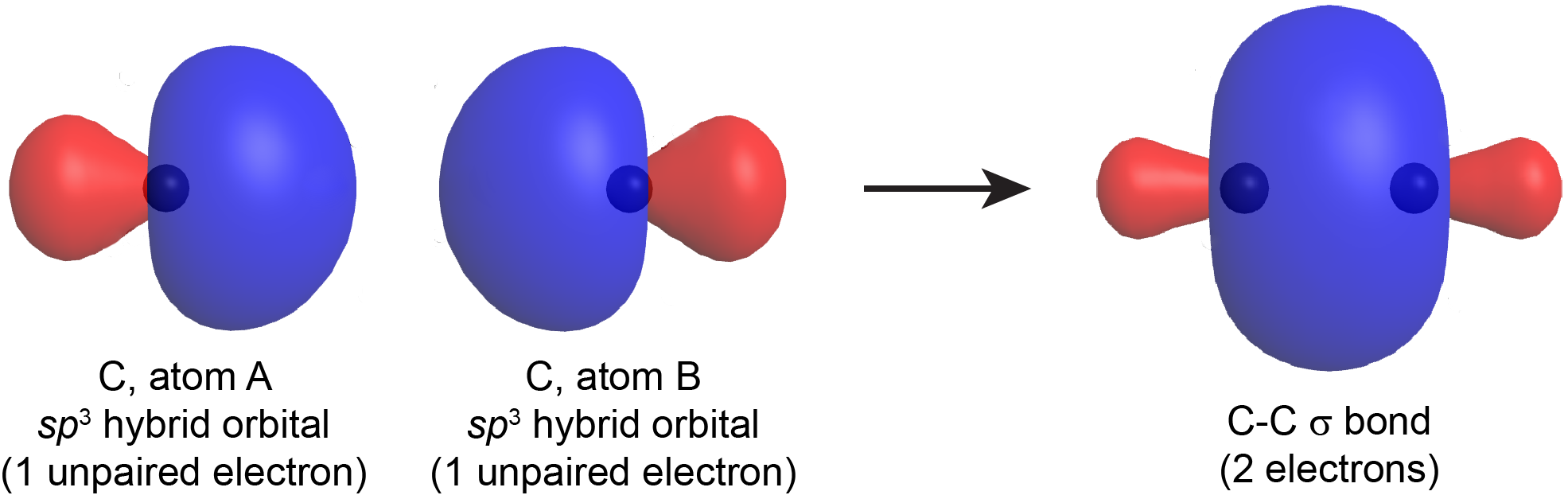

/chapter1/pages13and14/page13and14_files/chmo.png)