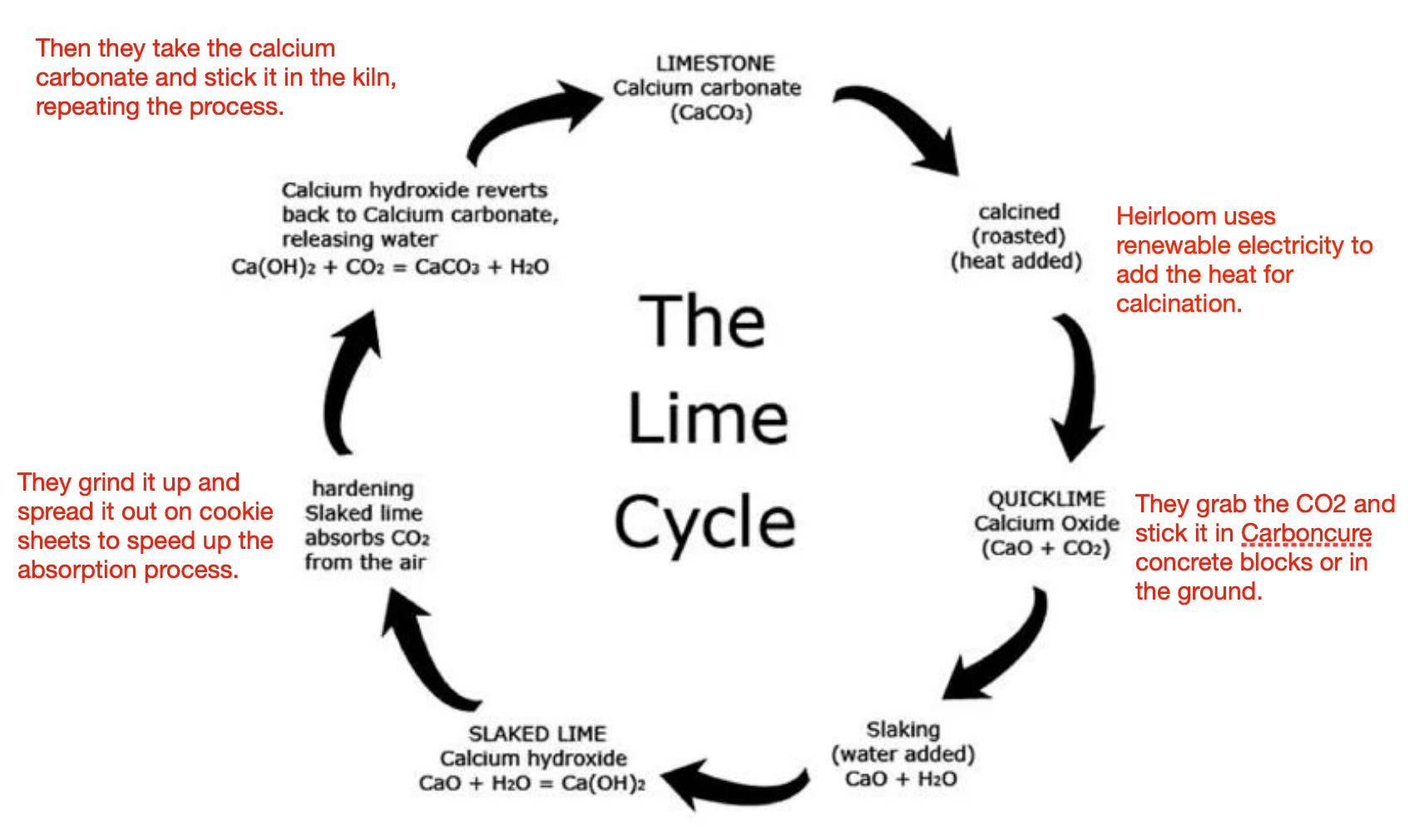
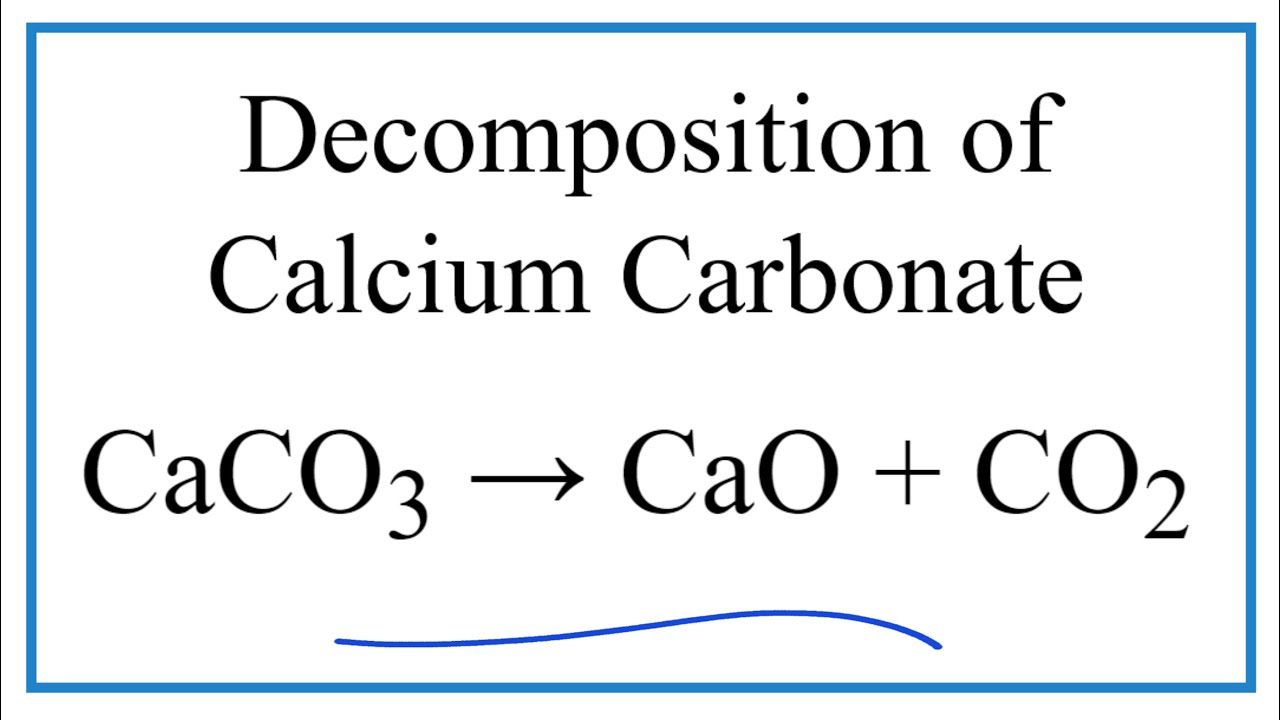
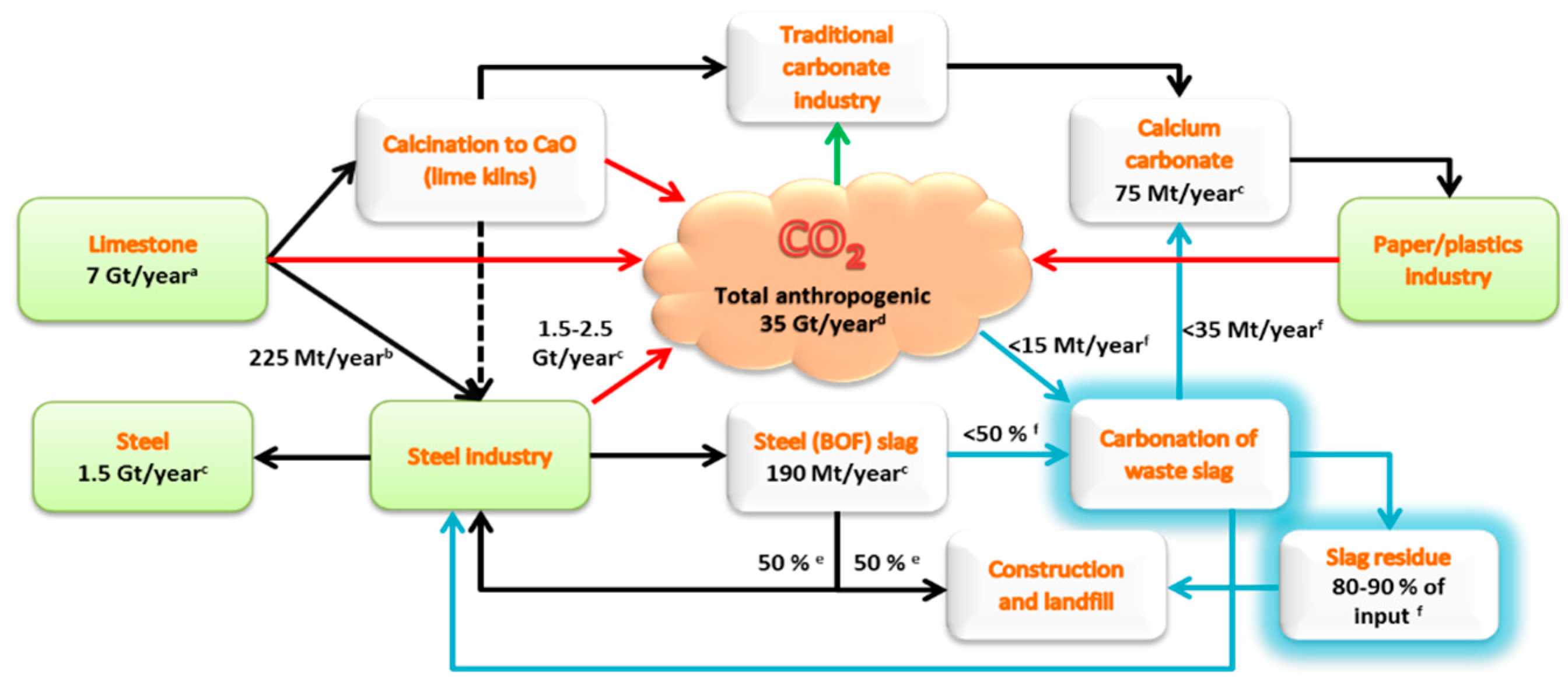
Case study for production of calcium carbonate from carbon dioxide in flue gases and steelmaking slag - ScienceDirect

Carbon dioxide conversion into calcium carbonate nanoparticles using membrane gas absorption - ScienceDirect

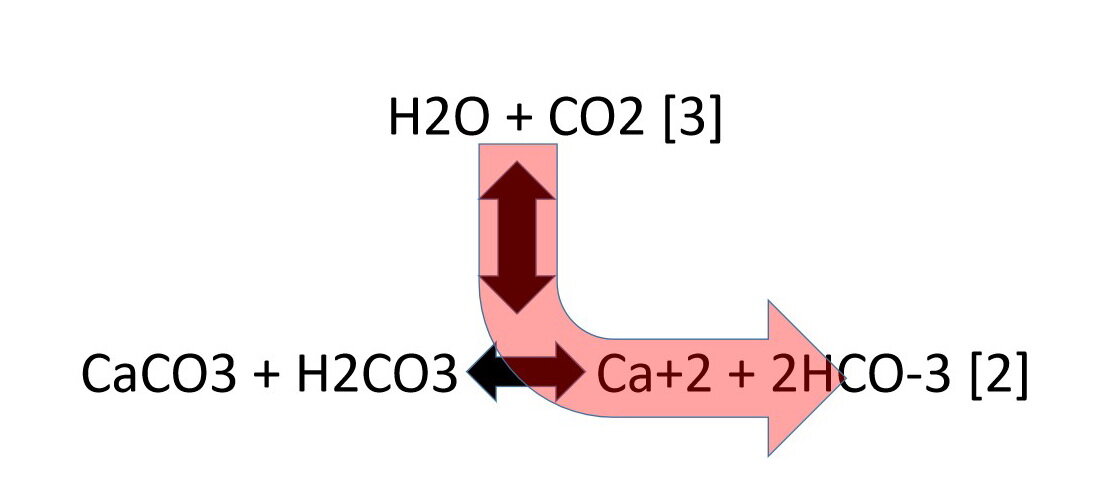
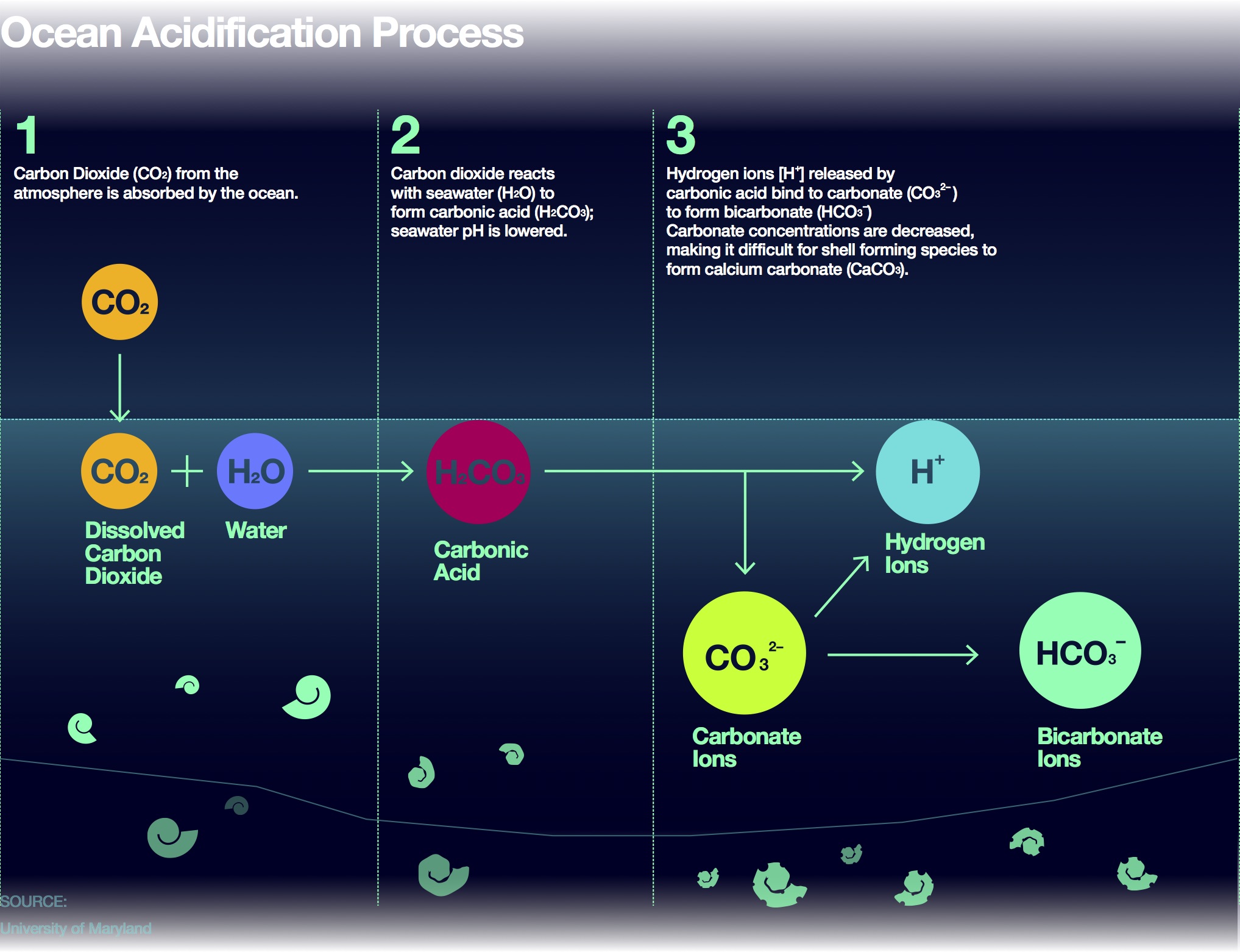
One method of determining the proportion of calcium carbonate in a coral is to dissolve a known mass of the coral in excess acid and measure the volume of carbon dioxide formed.
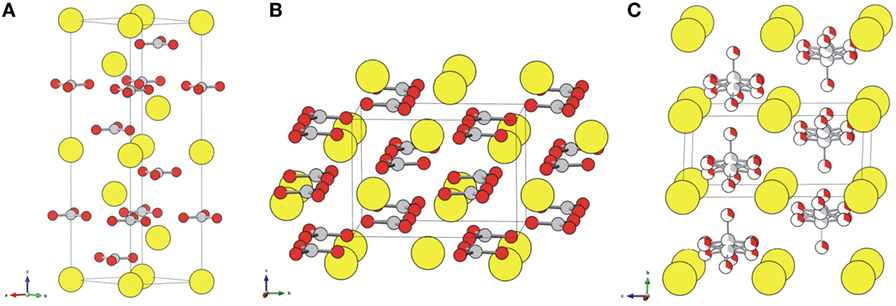
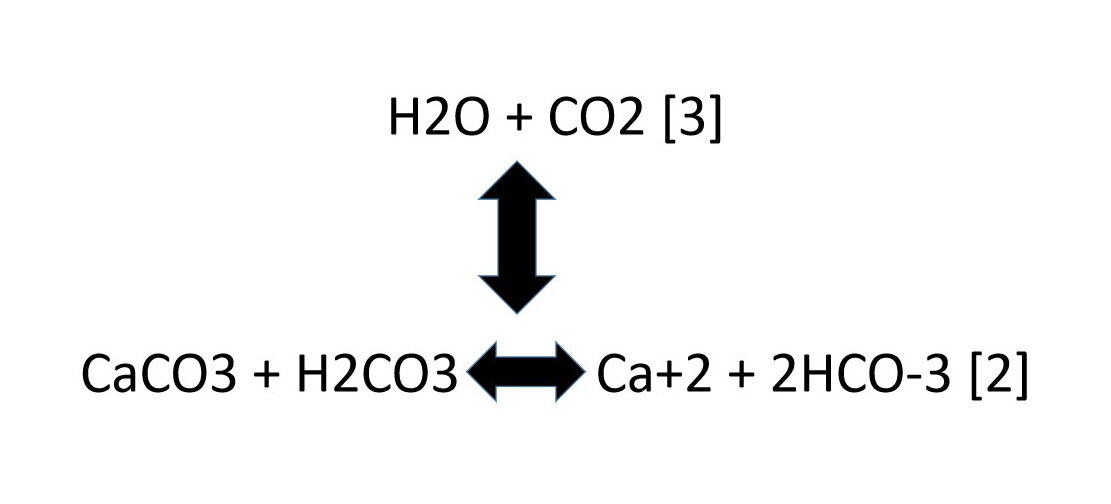
Frontiers | Calcium Carbonate Precipitation for CO2 Storage and Utilization: A Review of the Carbonate Crystallization and Polymorphism
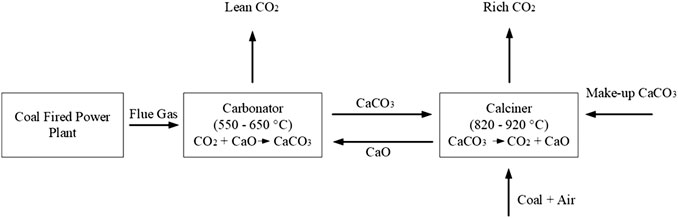
Frontiers | CO2 Capture for Dry Reforming of Natural Gas: Performance and Process Modeling of Calcium Carbonate Looping Using Acid Based CaCO3 Sorbent